New technology is quickly revolutionizing healthcare, but whether it is a wearable medical device, a surgical robot, respirator pump or a smart hospital, they have one thing in common: lives of patients are at stake. The medical industry responded with stringent industry regulations and standards for patients safety and security. As innovations progress, development time and cost are important factors.
“Logic Technology offers future-proof embedded development solutions for medical devices to meet these challenges.”
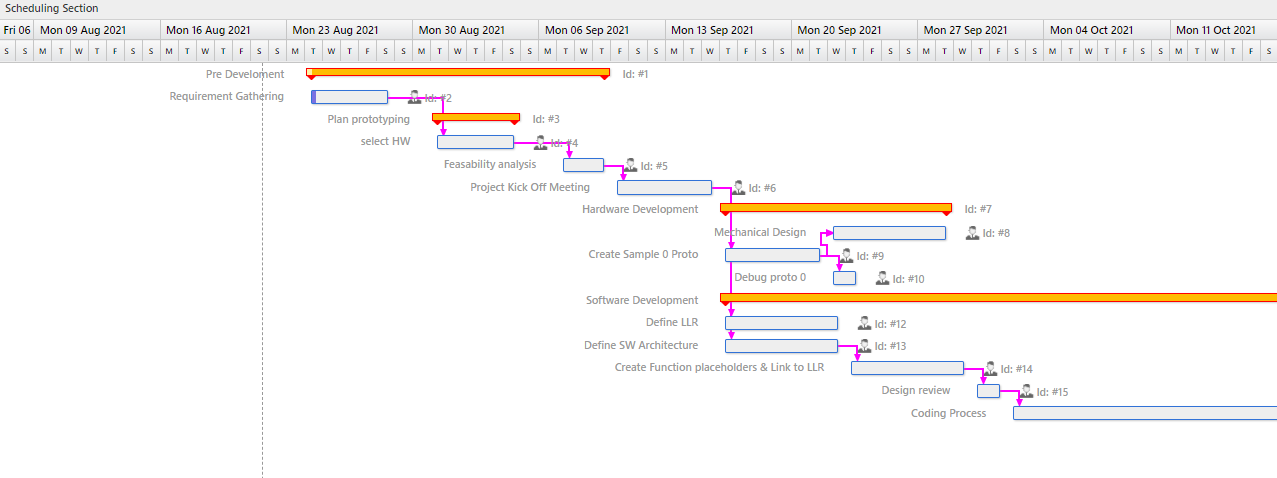
Accelerate development
Simplifying hardware development, speed up software build times, On-the-fly co-creating and swapping intuitive User Interfaces are some of the development aspects that can significantly lower costs.
Increase Reliability
No downtime, no data corruption, no programming errors at all, and still have room for flexibility.
Strengthen Security
With medical devices interpolating with other devices and systems, protecting patient information is of top importance. Software development with security in mind is key.
Ensure Compliance
As the use of open source in medical devices increases ,IP infringements lure, especially in a high competitive market. On top of that developing safe medical devices to the IEC 62304 standard is mandatory.
User Interfaces with high-reliability and ease-of-use
“How can we ensure reliability as well as ease-of-use for our medical GUIs?”
In order to keep pace with rapid innovations in patient diagnostics, collaborative care, and connected systems, medical device manufacturers recognize the need to deliver user experiences that streamline complex operations into clear and safe procedures. Whether it’s a portable monitor, infusion pump, or robotic surgery system, our Storyboard embedded GUI development framework is a proven solution for medical GUIs that require high-reliability and ease-of-use for practitioners. Storyboard connects UX designers and developers in a productive, parallel workflow that accelerates medical user interface design, development, and validation on low-power, low-cost platforms.
We help developers of safety-critical medical devices design secure GUIs without any compromises to UX through:
- Import directly from Photoshop, with layers, order, and names
- Animate objects easily with built-in animation timeline
- Add behavior to graphics quickly, without having to write code
- Deliver rich 3D graphics with support for Autodesk FBX, OBJ, and OpenGL ES
- Enforce clean architectural separation between GUI and application
User Interface design
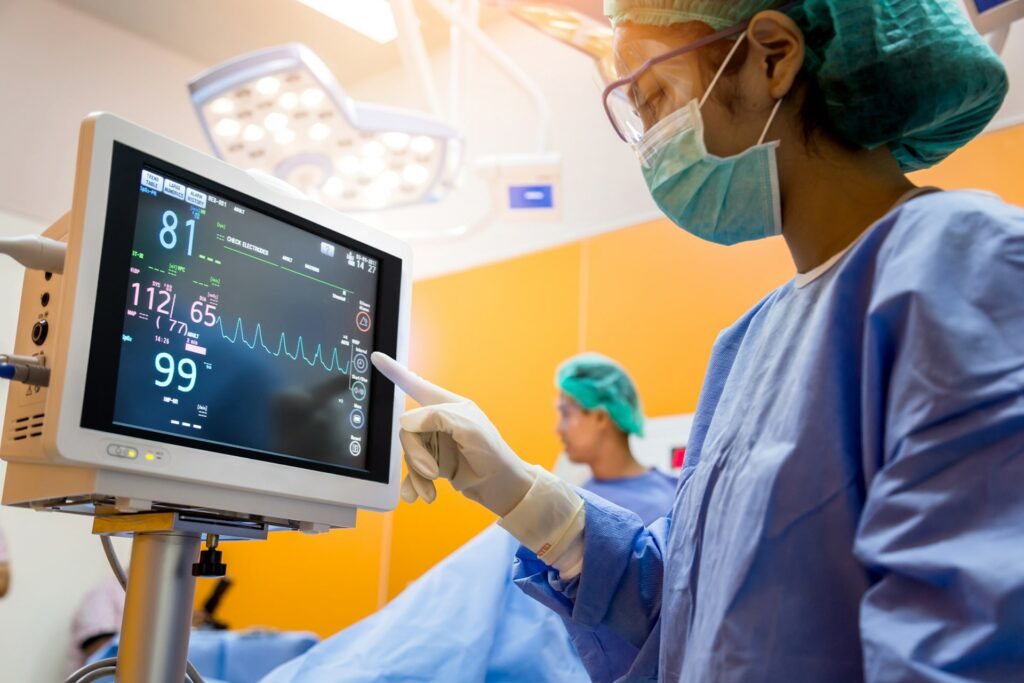
Increase code quality by cutting down compilation times
“How can we improve software quality and the time-to-market with our current resources?”
When developing medical devices, the quality of the code is the most important factor that needs to guaranteed. On the other hand, a quicker time to market could also mean the potential saving of lives. Meeting regulatory standards is one of the challenges Medtech developers face. By making better use of current resources, build compilation time can be cut down significantly, leaving time for more extensive testing and a quicker product launch.
By making use of all the idle CPU’s in a company’s cloud network, build compilations can be cut down by as much as 80%.
- Increase code quality
- Quicker time to market
- Efficient use of resources
Build Time Optimization
Ensure power-fail safety in your medical devices
“How to prevent data corruption due to sudden power loss or system crash in medical devices?”
According to the WHO, “Data integrity is the degree to which data are complete, consistent, accurate, trustworthy and reliable and that these characteristics of the data are maintained throughout the data lifecycle. The data should be collected and maintained in a secure manner, such that they are attributable, legible, contemporaneously recorded, original or a true copy and accurate.”
In medical environments many devices are designed for the purpose of collecting and processing patient data for various treatments and different reasons. One of them is that they provide a rich source of information that allows better decision making when doctors and nurses have access to it. The other one is that aggregating data from different areas allows facilities to see the bigger picture and finally, it can help derive insights on systemic wastes of resources within health institutions.
Manufacturers of such devices must therefore aim to develop devices which will protect critical patient data at any time during any scenario. Bearing in mind the consequences for the treatment of the patients when all vital data is lost or corrupted. The most common data corruption scenario’s are power outages and system crashes.
Logic Technology provides a power-fail safe file system which ensures 100% data integrity because it implements an atomic, transactional model where changes either occur in their entirety or not at all, which is to say, the file system always reverts to a “known good state” after a system crash or sudden power loss.
Additionally, we have an in-memory database management system designed to protect from loss of database availability, safeguarding data integrity and resistant to database corruption caused by application software defects. This database management system is designed for embedded devices which have to save and process large amounts of critical data fast and safe.
File Systems

Setup your development process to achieve medical compliance
“What is needed to comply to IEC 62304 and FDA standards?”
Medical device software – software life cycle processes is a standard which specifies life cycle requirements for the development of medical software and software within medical devices.
Developing safe and effective medical devices requires a balance of effective requirements management, risk mitigation, and automation.
We help developers of medical systems to organize a consistent development process for the entire product lifecycle up to the highest safety classification with future-proof embedded development solutions for medical software compliance.
Medical software compliance tools
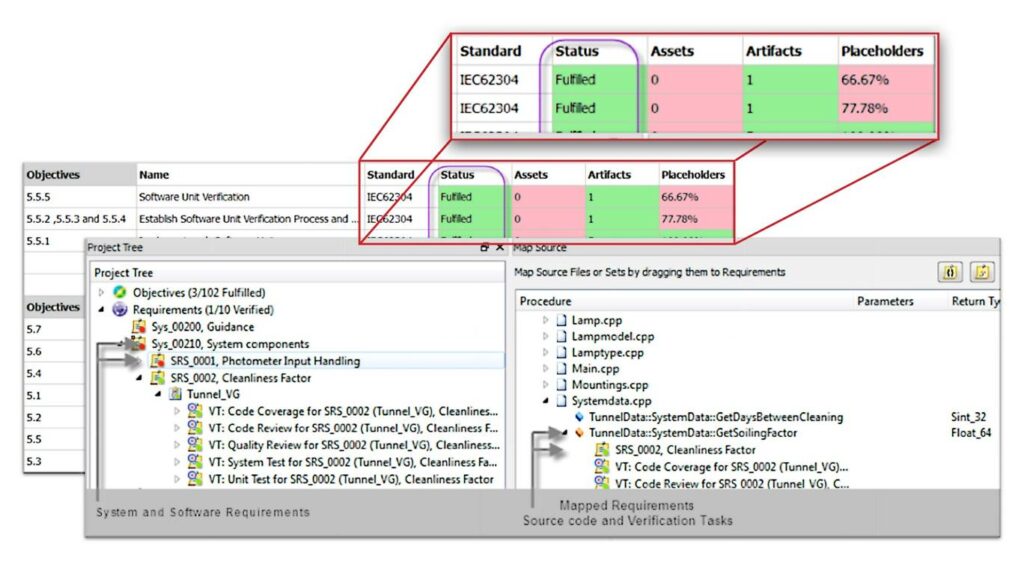
Include all stakeholders to create medical devices that meet your customers’ expectations
“How do we keep project management under control ?
It’s important to ensure safety from the start of development. Product testing isn’t enough to ensure patient safety. And patient safety is critical. Plus, building safety into your processes early on saves time and expense later
To comply with the safety standard, processes need to be well-documented, using software development tools can help you document and accelerate compliance.
We help to track coverage as well as establish relationships between requirements, use cases, test cases, defects and other related ALM items to achieve compliance and assuring on-time and better-quality delivery.
Medical software project overview tools